Explore Spine firsthand by downloading the Spine trial, free for Windows, Mac and Linux. All Spine Professional features are available in the trial except saving projects, texture packing, and exporting animation data, images and video.
File size: 64.5 MB Spine is an animation tool that focuses specifically on 2D animation for games. Spine aims to have an efficient, streamlined workflow, both for creating animations using the editor and for making use of those animations in games using the Spine Runtimes. Jun 27, 2012 Download Spine Pro III for iOS to sPINE PRO III HAS BEEN DEVELOPED IN PARTNERSHIP WITH RICHARD A. KUBE II, M.D., FACSS, FAAOS, CIME.PRESS REVIEWS:'The level of detail and anatomical.
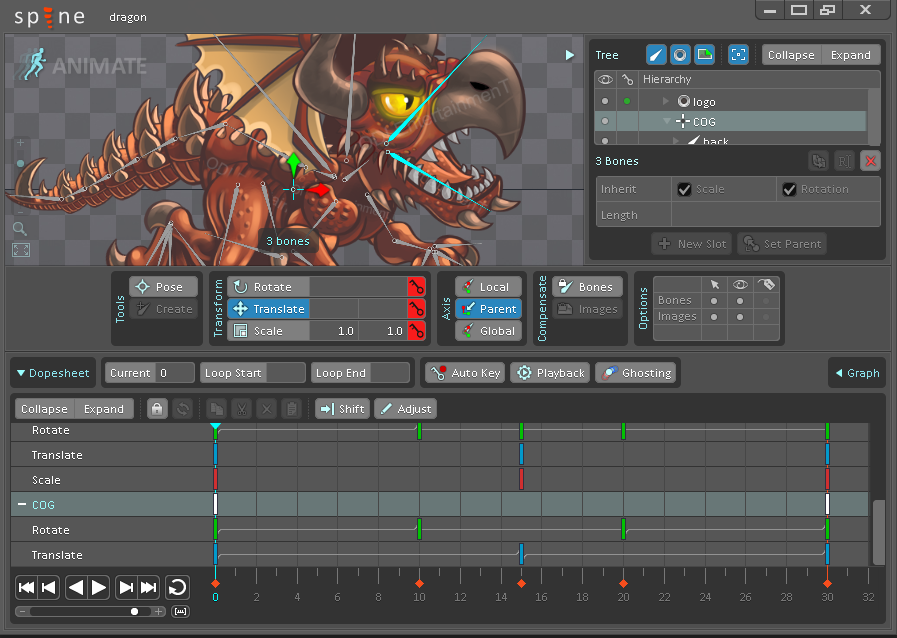
The trial comes with many example projects that include exported JSON animation data and texture atlases. These can be used to evaluate how the Spine Runtimes work with your game toolkit before purchasing.
To download Spine, choose the closest server and your operating system:
Note: The full version of Spine is downloaded from your personal Spine license page, received after purchase.
Purchase Spine

Support Spine development by purchasing Spine and enable save, export and use of the Spine Runtimes.

Here at Spine Pro Chiropractic, we have many different services to fit your specific needs. Whether you are experiencing back pain, feeling out of alignment, or are just looking to improve your. Find Spine software downloads at CNET Download.com, the most comprehensive source for safe, trusted, and spyware-free downloads on the Web. Crack The Spine Submissions. Download Spine 2.1.27 PRO (mac & win) Full (crack included) Spine is an animation tool that focuses specifically on 2D animation for games. Spine aims to have an efficient, streamlined workflow, both for creating animations and for making use of those animations in games. Spine is an animation tool that focuses.
This is a brief overview of information related to FDA’s approval to market this previously approved product for expanded use in two lumbar spinal levels. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: prodisc® L Total Disc Replacement
PMA Applicant: Centinel Spine, LLC
Address: 900 Airport Road, Suite 3B, West Chester, PA 19380
Approval Date: April 10, 2020
Approval Letter: Approval Order
What is it? The prodisc® L Total Disc Replacement (prodisc® L) is intended to replace damaged intervertebral discs in a patient’s lower back (lumbar spine). The prodisc® L consists of three implant components; two metallic (cobalt-chrome) endplates and a plastic (ultra-high molecular weight polyethylene) inlay.
This approval expands the indication for use of the prodisc® L to include treatment of up to two consecutive lumbar spinal sections (levels) from L3-S1.
How does it work? The prodisc® L is surgically implanted into the lumbar spine (L3-S1) in patients with degenerative disc disease in one or two consecutive intervertebral level(s). The device is intended to help restore the natural distance between two vertebrae and the natural motion of the lumbar spine.
When is it used? The prodisc® L is indicated for spinal disc replacement surgery patients who:
Spine 2d Pro Free
- Are skeletally mature and have no spinal growth remaining.
- Have a condition in which pain is caused by wear-and-tear on a spinal disc (Degenerative Disc Disease or DDD) at one or two consecutive levels in the lumbar spine from L3-S1.
- Have no more than 25% of the affected vertebral disc that has slipped forward out of the proper position (Grade 1 spondylolisthesis).
- Have no relief from pain after at least six months of non-surgical treatment.
Spine Pro Free Download
What will it accomplish? The prodisc® L is used to replace a damaged intervertebral disc. The device may repair disc height, may reduce pain, and may allow movement at the level where it is implanted. A clinical study of the device showed that the prodisc® L was at least as effective as spinal fusion for treating patients with Degenerative Disc Disease (DDD) for at least five years past treatment. Patients with two-levels of prodisc® L experienced a lower rate of secondary surgery than two-level spinal fusion patients.
When should it not be used? The prodisc® L should not be implanted in patients with the following conditions:
Spine 2d Pro Free Download
- Infection at the implant location or elsewhere
- Osteopenia or osteoporosis with a T-score (bone density measurement) of less than -1.0
- Narrowing in the lumbar bones that puts pressure on the spinal cord and nerves (spinal stenosis)
- Allergy or sensitivity to implant materials (cobalt, chromium, molybdenum, polyethylene, titanium)
- Compression syndromes due to a herniated disc
- Stress fracture in the lumbar spine
- Affected vertebral endplate that is smaller than 34.5mm when measured from the middle of the disk to the side of the disc and/or 27mm when measured from the front of the disc to the back of the disc
- Damage to the affected disc due to current or past damage
- Spondylolisthesis that is geater than Grade 1
Additional information (including warnings, precautions, and adverse events):
Proxfree
Spine Pro Free Download
Regulated Product(s)